The business community’s new exercise that is taking over lately is “connecting the dot game”.
Drugs Intelligence
Data-Driven Solutions
The DI company has developed an exclusive technological platform, The Compass that aggregates unique data sets and applies manipulation, unification and deployment of the data to BI using AI and ML models.
Vision and Overview

Vision
Our company’s vision and focus are based on Dr. Shlomo Sadoun’s thesis “Six pillar of successful launches of orphan drugs globally" utilizing cutting edge technologies to support smart business decisions that will lead to better healthcare solutions.
Overview
- Our unique platform allows us to extract all relevant Data identifying the most lucrative data set or article using advance technologies.
- Collect historical data, risk factors and causes data using ML allows the model to predict prevalence values based on systematic research analysis validated by designated scientists.
- Monitor markets and collect real-time intelligence and Rare Diseases Data.
- Making Data-driven decisions and creating business opportunities.


The Compass
The compass essence is to guide orphan drugs companies towards excellent launch of their orphan drugs, based on the orphan drug 6 pillars of success methodology. The Compass providing eagle eye view of the orphan drug market opportunities in 65 countries and counting, with a mission of answering unmet needs for patients and their caregivers dealing with rare disease.
Successful Launching of Orphan Drugs Methodology
Detection and diagnosis
Treatment access incentive and reimbursement
Key opinion leader and healthcare professional collaboration
Treatment retention and collection of data & real-world evidence
Advocacy group alliances
Disease Awareness and education
Six Pillars of Success
Learn more about the revolutionary Six Pillars methodology of successful launching an Orphan Drug Globally
1. Detection and Diagnosis - "Newborn Screening"
Detection and diagnosis is performed by Drugsintel's orphan prevalence software, which provides the biggest orphan drug prevalence data in the world together with a smart machine learning and AI prediction tool. DrugsIntel detects and collects genetic testing data for newborn babies and pregnant women to better identify rare disease patients as early as possible. Cross-matching various diagnostic techniques, solutions, and their successful implementation in relevant clinics holds great value. The activity is digitalized and automated, in order to maximize user experience.
Newborn Screening
- This strategy has the potential to achieve early diagnosis of rare diseases and apply mitigation interventions that can save and/or prolong lives if provided early enough in the process.
- Multiple conditions can be identified from a single bloodspot data collected at birth.
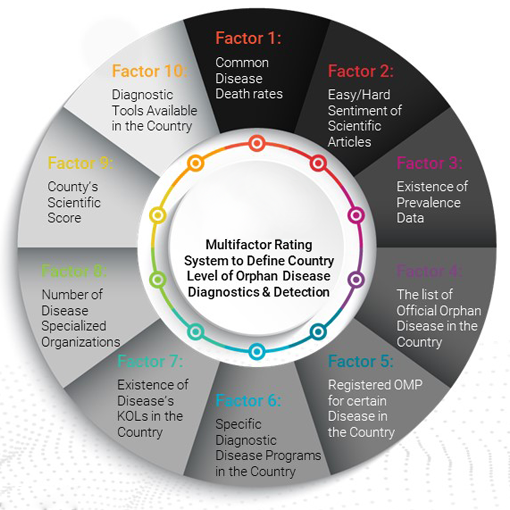
2. Treatment access incentives and reimbursement
DrugsIntel today provides robust data on reimbursement and incentives of various rare diseases by country and globally. The collected data provide important information at a glance, with trends and clusters as a support to business decisions. Automatic screening of treatment access availability will be applied through the engagement of unique data sources providing robust information. The data are territory-related in order to better understand patterns and patient distributions.

3. Key opinion leader and healthcare professional collaboration
DI team of analysts use different engagement tools to identify KOL and HCP. Combining various platforms gauge valuable information on the two groups (mainly KOL). Leveraging the platforms, ensure laser focus reach to the most important leading physicians and researchers for a given orphan disease.
4. Treatment retention and collection of real-world data
Our Team of Experts continuously monitor and collect Real-World Data that provide meaningful insight which can be impactful to the KOL, HCP and pharmaceutical companies education. This activity can directly contribute to the orphan products acceptance and sales prediction. Using numerous validated sources, consisting of clinical trials data, healthcare websites and blogs, together with DrugsIntel's unique NLP algorithm can result in meaningful outcomes. Making sure to engage in the patient journey by applying a patient-centric approach, can systematically improve the treatment outcomes.

5. Advocacy group alliances
DrugsIntel is building a database silo containing the vast majority of advocacy groups, cascaded by their importance to a certain disease. Having a live stream communication with those organization, following their conferences and special disease days can contribute to the overall orphan disease coverage. Additionally, collaboration which include data sharing, KOL & patients communication, can result in impactable outcomes.

6. Disease awareness and education
Using various technological tools, to better evaluate and position a given orphan disease. Our specialized, qualified team of experts will perform a full education and awareness plan to communicate the disease to the relevant care givers and stakeholders with an aim to constantly enlarge the solution outreach. Advocating the disease awareness and education can result in more patients diagnosed or aware of their own or beloved ones disorder. It takes about 7 years and approximately 10 different wrong diagnoses to reach an optimal diagnosis. Suffering from an orphan condition can be debilitating. Building communities and knowledge around the disorder can contribute to an investment in research and development and pressure on governments to provide incentives and reimburse certain orphan diseases.
Our Services
DrugsIntel customers can use our Compass to address and solve unique challenges in the Orphan Drug and Rare disease world
Compass Dashboard
Unique custom design and personalized dashboards with information about Orphan Drugs and Rare diseases
Fast Respondent
Drugsintel professional team of experts and an analyst online support
Portfolio Management
Build a smart, data-driven Orphan Drug portfolio management system based on our Six Pillars methodology
Monitoring
Monitor and collect real-time intelligence on Global Rare diseases and Orphan Drug market opportunities.
The Executive Team
Our Team

Dr. Shlomo Sadoun
Co-Founder & ChairmanShlomo carries over 13 years of strategic planning and management experience within the pharmaceutical industry. Shlomo holds a Master's degree in Global Management by Salford University (UK), and a Doctorate in Business Administration from OUS University (Switzerland).

Elad Levy Lapides
Chief Executive OfficerElad brings over 20 years of experience in strategic planning, operations, and business development. He holds an International MBA from Bar-Ilan University and has specialized in negotiation at Harvard Business School. As a tech-oriented manager with vast experience in development, Elad has a proven track record of driving business growth, leading cross-functional teams, and managing complex projects. His leadership is characterized by a dynamic approach, strong execution skills, and a commitment to fostering a culture of continuous improvement and innovation.

Doron Azran
Chief Product OfficerDoron brings extensive expertise in developing advanced technological tools. As CPO of DrugsIntel, he led product strategy and established key partnerships, guiding the company through crucial growth stages. He coordinated cross-functional teams and engaged with vendors to strengthen the market position. Under his leadership, DrugsIntel developed precision medicine platforms that aggregate and process data using AI and ML models. Doron also developed The Obesio, Alzimind, and Supply Chain platforms, driving innovation and business growth.

Dr. Vladimir Levin
Strategic Business Development ManagerVladimir carries over 15 years and more than 40 successful full-cycle projects in the field of marketing, technical transfer and R&D in the background. Skilled in R&D, BD and Marketing Management, Pharmaceutics, Business Planning, and Market Research. Experienced professional with marketing and medical education. Vladimir M.D. in General Studies from Moscow Institute of Medical and Social Rehabilitation (RU) and M.Sc in Pharmaceutical Marketing and Management from Russian Presidential Academy of National Economy and Public Administration (RU).

Alexander Lasorunskiy
Data ScientistAlexander carries over 10 years of experience as an analyst. Prior to joining the group, Alex served as a business analyst for the leading retailer and as a financial analyst for the private wealth management company. Alexander holds an MA in Business Management from Odessa State Economic University (UA) and MA in Statistics from Haifa University (IL).

Andrii Rapko
DBA/Full Stack Software DeveloperAndrii carries over 5 years of experience as a ERP software developer, 2 year as DBA, 3 years Full-Stack Developer. Prior to joining the group, Andrii served as full stack web developer at Inetex LTD (IL) and ERP software developer and business analyst at LLC Business Evolution (UA). Andrii holds an MSc from West Ukraine National Technical University (UA).
Careers
Want to be a part of HI-TECH family, come work with us!
Appointed Pharmacist
- Registering the company’s pharmaceutical products.
- Maintaining of the registration licenses throughout the product life-cycle.
- Submissions of new product registration, registration renewal, variations and deregistration applications of a pharmaceutical product to the Pharmaceutical Administration and Standards Institute of the Israeli Ministry of Health.
- Leaflet and packaging materials creation and maintenance.
Send your cv to: 𝗷𝗼𝗯𝘀@𝘀𝗸-𝗽𝗵𝗮𝗿𝗺𝗮.𝗰𝗼𝗺

Regulatory Affairs Associate
- Manage and support regulatory activities required for submissions to Israeli MoH.
- CMC post-approval regulatory activities of the company.
- Support worldwide suppliers to meet Israeli MoH regulatory requirements for variations and renewals submissions.
- Ensure compliance with internal procedures and Israeli regulations and standards.
- Maintain regulatory records and other controlled documents.
- Other Regulatory and Quality duties as assigned.
Send your cv to: 𝗷𝗼𝗯𝘀@𝘀𝗸-𝗽𝗵𝗮𝗿𝗺𝗮.𝗰𝗼𝗺

Recent Updates
News
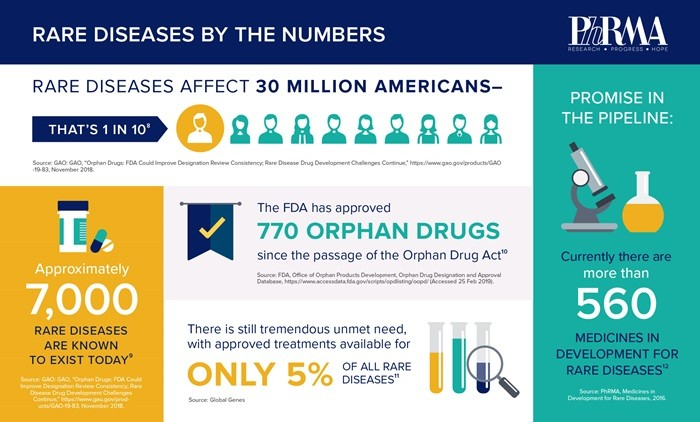
Orphan drugs impact about 7% of the global population, with 30M cases in the US and about 40M cases in Europe.
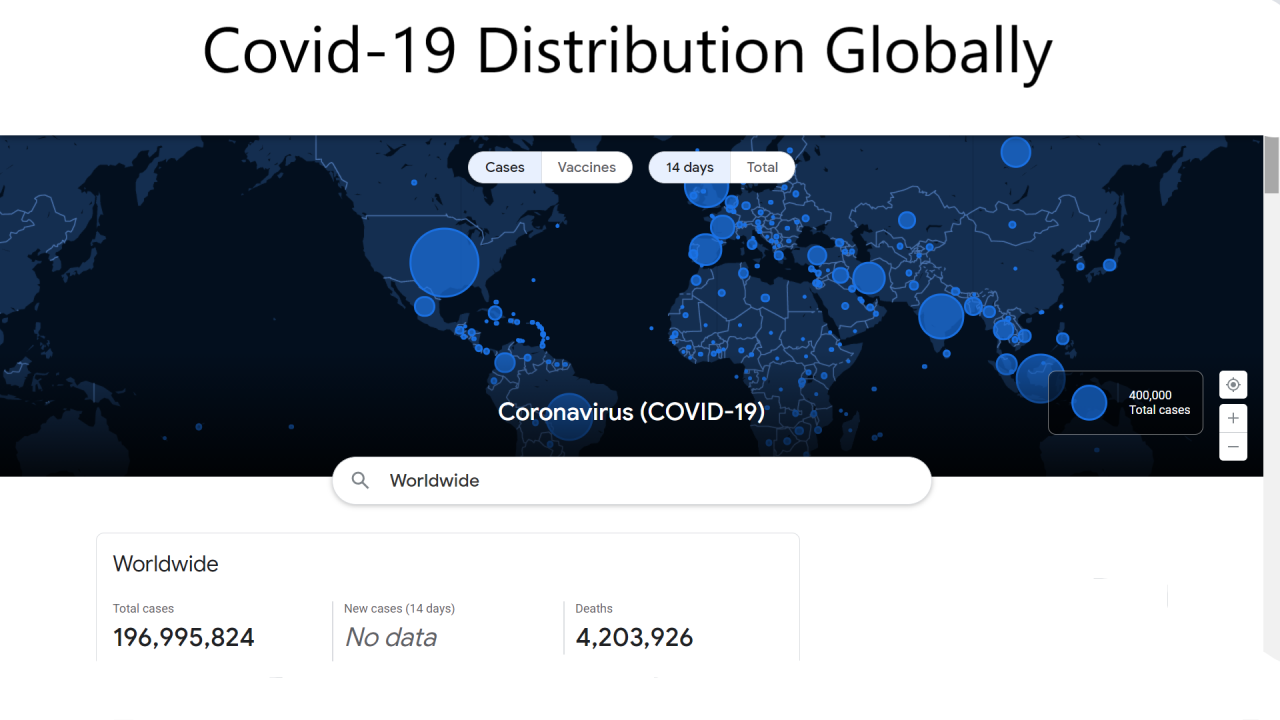
It’s almost August 2021, and we are 20 months into the Covid-19 pandemic that altered in so many ways.

Many companies are applying various methodologies aiming to lead the generic pharmaceutical sphere.
DrugsIntel to provide life saving insights DrugsIntel platform had proved to be extremely efficient during the covid19 pandemic outburst.




