Orphan Drugs - from Neglected Diseases to a Global Mission
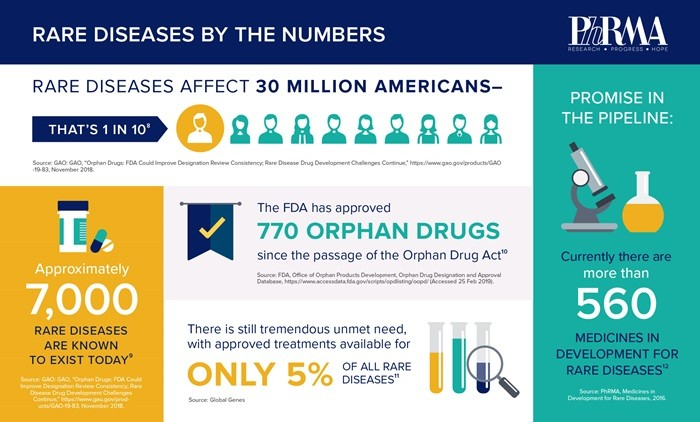
Orphan drugs impact about 7% of the global population, with 30M cases in the US and about 40M cases in Europe. 80% of orphan diseases are genetic by their origin and over 50% affect children. As of date, merely 12% of the 7000 orphan diseases have an indicated therapy. There are about 20-25 new orphan New Therapeutics Entity (NTE) drug approvals, which count as 40-50% of the new drug approvals in the US a year. Orphan drugs Compound Annual Growth Rate (CAGR) of sales keep being vigorous in the average rate of 12% y/y with the same growth rate expected through 2025. About 50% of the companies developing orphan drugs are Small and Medium Enterprise (SME), with limited resources both monetary and manpower wise. The SME’s are usually developing a certain drug that will make it to the finish line, with an aim in most cases to sell the drug to a big pharma giant. A mere handful number of companies will try to develop an orphan franchise focusing on a specific rare condition, clustering their pipeline & development around this area (e.g., Vertex - SF, Alexion pharma – BD). Disease awareness and advocacy groups made tremendous development in the last decade, putting orphan diseases in the global spotlight. Years of hard work paid off, where the general population applies empathy to patients and caregivers suffering from a rare disease. It is widely common to see expensive drugs treating ultra-rare diseases covered by insurance companies and governmental budgets. Orphan drugs, in general, are categorized into 3 categories ultra-orphan with 1 in 1,000,000 patients or less, a rare orphan with 1 in 100,000 or more, and orphan with 1 in 10,000 patients or more up to the orphan definition trash hold of 5 to 10,000 in Europe and alike globally. There is a direct correlation between the rarity of the disease and the treatment cost. As rare the disease is the higher is the cost per treatment, as companies need to recover their investment and risk bringing the product to the market. The additional interesting factor is the successful and impactable output of the treatment that plays a vital role in drug reimbursement and pricing. Gene therapy product as Zolgensma for SMN gene correction treating fatal patients with Spinal Muscular Atrophy (SMA), that in most of the cases before Zolgensma won’t make it to their 3rd year birthday, now have a revolutionary treatment that completely corrects the defect gene and restores those vulnerable patients and their caregivers to a normal life. Nevertheless, the treatment counts as one of the most expensive treatments as of date with a tag price of $2.1M per infusion, way above the affordability of most people. The human genome discovery is and will drive new and fascinating treatments that are gene-related, to treat many of the orphan conditions, that just as a reminder 80% are gene-related diseases. Today more than ever it is imperative to give to the pioneer in rare disease development all the support they need. I am positive that with the development of more orphan drug companies and the increased competition in the field the prices will naturally go down to a more affordable level. At this stage, it is part of orphan drug’s evolution to having orphan drugs predominantly present by high prices. There are numerous technics to fund those costly treatments, with a special global fund to support ultra-orphan and rare orphan diseases, country special fund to finance orphan diseases as we can see evolve in Russia, Serbia, Saudi Arabia, and more countries. I believe it is of the mutual benefit of all stakeholders to be a part of the initiative to encourage innovation especially in the orphan space and to contribute directly to the overall mission to save lives. As a reminder, 50% of orphan disease cases attribute to children, that can be given life for a better mutual future. Hence, orphan diseases are an impactable theme that needs to be handled as a global mission for the sack of humanity.
References:
Dr. Shlomo Sadoun:
Chief Executive Officer at SK Pharma | Co-Founder & Chairman at DrugsIntel
https://www.linkedin.com/in/dr-shlomo-sadoun-5a72586/
Article:
https://www.linkedin.com/pulse/orphan-drugs-from-neglected-diseases-global-mission-sadoun/